Compounds and Mixtures
A compound is a substance that contains two or more elements chemically combined together. A mixture is something that contains two or more elements not combined chemically. It is always difficult to identify a mixture from a compound. Before going any further into this topic, let us start by looking at the differences between compounds and mixtures.
A Binary Compound.
Is a compound is a substance that contains two or more elements chemically combined together. This is a very important difference from mixtures. Mixtures can contain more than one element but the elements are not chemically combined. The number of chemical substances known is approximately four millions.
All compounds on earth are made from about one hundred simple materials. Such compounds range from simplest substances, like water, which contains only two elements, to those complex materials of which our own bodily tissues are composed.
The following is a short list of common compounds and the elements they are made of.
The following is a short list of common compounds and the elements they are made of.
Compounds have different properties from the elements that make them up.
For example:
1. Water (H2O) is a colorless liquid at room temperature but the elements that make it, hydrogen and oxygen are both gases.
2. Sodium chloride is a white solid made of sodium and chlorine. Sodium is a solid, highly reactive metal, and chlorine is a greenish yellow gas with a chocking smell.
The Concept of a Mixture
A mixture is something that contains two or more substances not combined chemically. The substances may mix up completely or they may remain separate.Our environment is a mixture of all forms of matter. For example, the earth's crust is a mixture of soils, rocks, minerals, and water. Sea, river, and lake waters contain dissolved gases, living organisms and, sometimes, salt. Air consists of gases, water vapor, and dust particles.
The components of each of these mixtures could be elements such as oxygen, nitrogen, Sulphur or gold. Alternatively, the mixture might consist of elements and compounds such as hydrocarbons (e.g. petroleum), water, metallic oxides or salts.
Other substances that can form mixtures when placed or mixed together include sand and sugar, maize and bean seeds, soil and table salt, water and mud, etc.
Mixtures into Solutions, Suspensions and Emulsions
Classification of mixturesMixtures can be classified as solutions, suspensions or emulsions. This classification is based on whether the mixed substances dissolve completely or not. It also depends on the nature of the mixtures that result upon mixing. Let us look at each category in detail.
Solutions
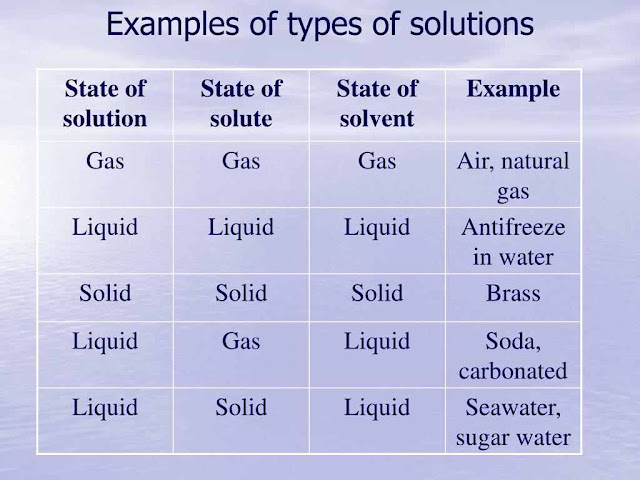
A solution is a uniform mixture of two or more substances. Such mixtures may be a solid in a liquid, a liquid in a liquid, a liquid in a gas and, very rarely, a gas in a gas. (See table below). We most often think of a solution as being made of a solid dissolved in a liquid. For example, solutions of sugar or salt in water are quite common.A solid that dissolves in a liquid is called a solute while the liquid in which that solid dissolves is called a solvent. For example, sugar and salt are solutes and water is a solvent. However, other substances that are not normally solids can be found dissolved in a liquid. For example, the gases, carbon dioxide and oxygen, dissolved in water are important for life to continue in oceans, seas, lakes, rivers, etc.
Less obvious perhaps, but quite common, are solutions of one liquid in another. Alcohol mixes (dissolves) completely with water. Beer, wine and whisky do not separate into layers of alcohol and water (even when the alcohol content is quite high). Alcohol and water are completely miscible, that, is they make a solution.
Solutions of gases in gases are very uncommon. Technically, air could be described as a solution of several gases in nitrogen, though this could be unusual everyday use of the term. However, it is interesting to note that different gases always mix completely with each other.
Examples of types of solutions
Suspensions
A suspension is a cloudy mixture of solid particles suspended in a liquid. A solid is said to be in suspension in a liquid when small particles of it are contained in a liquid, but are not dissolved in it. If the mixture is left undisturbed, the solid particles will slowly settle to the bottom of the containing vessel, leaving the pure liquid above them.Muddy water is a typical suspension. The mud would settle after a time if left undisturbed leaving brown residue on the bottom of the containing vessel and clear water above. The particles of mud would be retained by filtering whilst the water (and any solids in solution) would pass through.
If you mix flour or chalk dust in water, it forms a suspension. Their particles are simply dispersed (spread) throughout the water and would eventually settle down to the bottom of the vessel if left undisturbed for sometime.
Differences between solutions and suspensions
|
Solutions |
Suspensions |
|
|
|
|
Homogeneous |
Heterogeneous |
|
|
|
|
Transparent/clear |
Opaque/not clear |
|
|
|
|
Particles completely dissolved |
Particles separate on standing |
|
|
|
|
Components separated by evaporation |
Components separated by filtration |
|
|
|
Emulsions
An emulsion is a cloudy mixture of tiny droplets of one liquid suspended in another liquid. Sometimes two immiscible liquids will not separate out into two layers when mixed together. One of the liquid may form droplets and spread throughout the other to form an emulsion.Cooking oil and water do not mix but they will form an emulsion when they are mixed and shaken. Droplets of oil will spread throughout the water. Unlike pure liquids, emulsions are cloudy (opaque). So you cannot see through them. The emulsion will not settle like a suspension.
Which other liquids you know can form suspensions?
Formation of mixtures
Mixtures can be formed from different substances in two major ways.The first type constitutes homogenous mixtures, where the substances are totally mixed together uniformly. Examples include solutions of salts and sugars in water.
The second type constitutes heterogeneous mixtures, where the substances remain separate and one substance is spread throughout the other as small particles, droplets, or bubbles. All emulsions and suspensions fall under this category. Examples include suspensions of insoluble solids or oil droplets in water.









0 Comments